When restoring a corporation, it's essential to set the confirmation day that was owing prior to it was struck off. In case you’re not sure of the proper day, Make contact with Corporations Household in advance of filing your confirmation statement (form CS01).We’d love to set added cookies to understand how you use GOV.United kingdom, keep in
The best Side of process validation protocol template
CSV may be costly and time-consuming, specially if you’re validating on paper and haven’t adopted a threat-primarily based approach to find out the suitable amount of tests and documentation necessary to meet regulatory anticipations. The FDA's Normal Basic principle of Software Validation Assistance outlines these expectations. Assembly regul
validation of manufacturing process for Dummies
To learn more about the World Lender classification system, make sure you Simply click here. Now incorporates the subsequent nations, except where Worldwide sanctions use:One of the better ways to effectively perform and keep track of your GMP Validation is by digitizing the process. Digitized processes will help you validate GMP processes much fas
A Secret Weapon For why 70% IPA
05% – 6% concentrations. Trace metals and Other folks contaminants lessen its balance, as does daylight. When blended with acidic substances which include other cleaners or ammonia, a harmful chlorine gas forms. Bleach should generally be utilised with consideration for suitable protecting tools and air flow.Cleanroom amenities typically appear t
mediafill test in sterile manufacturing - An Overview
This summary shall be up-to-date after Every new APS is full. The summary shall incorporate a table with the following info, in a minimum amount:Evaluation and explore the historical sterility good outcomes through the similar product or service or filling line Considering that the last effective media simulation.Internet site procedures shall be d
 Tatyana Ali Then & Now!
Tatyana Ali Then & Now! Val Kilmer Then & Now!
Val Kilmer Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now!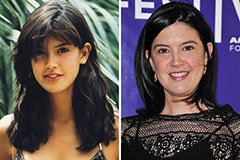 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Bill Murray Then & Now!
Bill Murray Then & Now!